

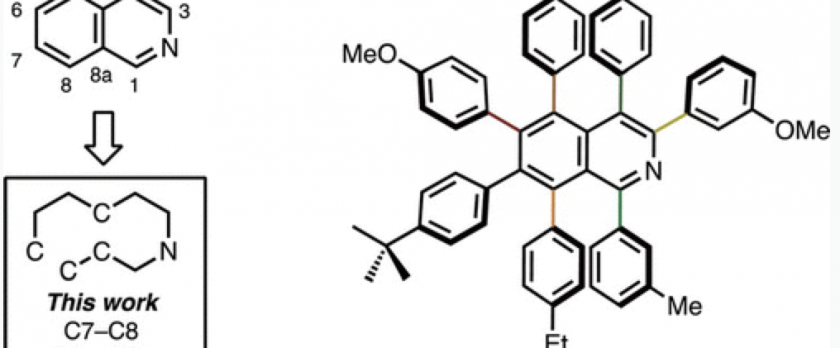
Synthesis of A Heptaarylisoquinoline: Unusual Disconnection for Constructing Isoquinoline Frameworks
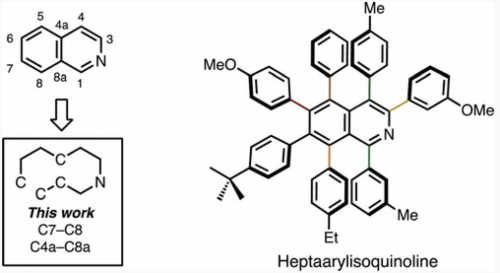
In a novel disconnection of isoquinoline ring synthesis at the C7–C8/C4a–C8a bonds, these bonds can be formed by a [4+2] cycloaddition between thiophene S,S-dioxide and alkynes. With a subsequent C–H arylation of the resulting hexaarylisoquinoline at the C3 position, the synthesis of a fully substituted isoquinoline has been achieved.