

S. Kirchberg, S. Tani, K. Ueda, J. Yamaguchi, A. Studer, K. Itami, Angew. Chem. Int. Ed. 2011, Early View. DOI: 10.1002/anie.201007060
It adds up to 4!
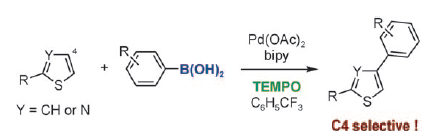
Thiophenes and thiazoles can be arylated in the 4- rather than the expected 5-position in a new CH functionalization reaction (see scheme; TEMPO: 2,2,6,6-tetramethylpiperidine-N-oxyl). The boronic acid proved to be the key to achieving the otherwise difficult C4 selectivity. The method was applied to a concise synthesis of a key pharmacological structure with potential for treatment of Alzheimer’s disease.