

Masakazu Nambo, Yasutomo Segawa, and Kenichiro Itami
J. Am. Chem. Soc. 2011, ASAP. DOI: 10.1021/ja111213k
Controlled Synthesis of a Range of Functionalized Fullerenes
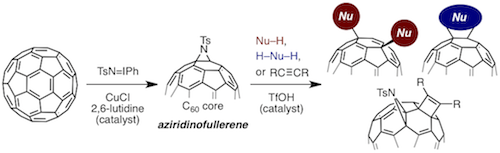
An aziridine moiety on the fullerene core can serve as an acid-triggered reacting template for the controlled synthesis of a range of functionalized fullerenes that are otherwise difficult to synthesize in an efficient and selective manner. A copper-catalyzed aziridination of C60 for the practical synthesis of aziridinofullerene and acid-catalyzed reactions of aziridinofullerene with mono- and bifunctional nucleophiles as well as alkynes are described. The rapid generation of structural diversity in a single chemical operation using the common platform is notable.