

π-Extended Rubrenes via Dearomative Annulative π-Extension Reaction
Wataru Matsuoka, Kou P. Kawahara, Hideto Ito*, David Sarlah, and Kenichiro Itami*
J. Am. Chem. Soc. 2023, 145, 658–666. DOI: 10.1021/jacs.2c11338
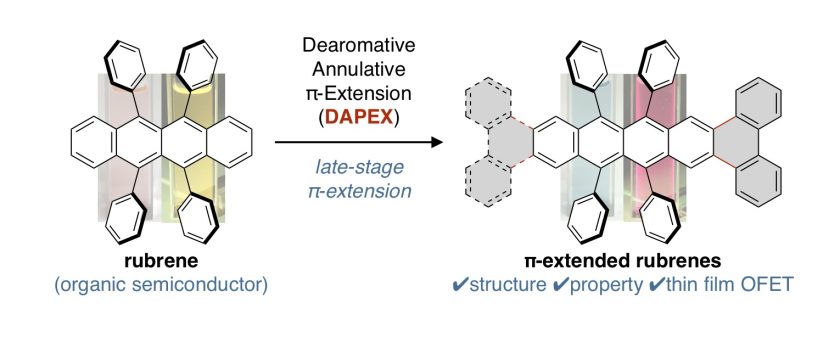
Among a large variety of organic semiconducting materials, rubrene (5,6,11,12-tetraphenyltetracene) represents one of the most prominent molecular entities mainly because of its unusually high carrier mobility. Toward finding superior rubrene-based organic semiconductors, several synthetic strategies for related molecules have been established. However, despite its outstanding properties and significant attention in the field of materials science, late-stage functionalizations of rubrene remains undeveloped, thereby limiting the accessible chemical space of rubrene-based materials. Herein, we report on a late-stage π-extension of rubrene by dearomative annulative π-extension (DAPEX), leading to the generation of rubrene derivatives having an extended acene core. The Diels–Alder reaction of rubrene with 4-methyl-1,2,4-triazoline-3,5-dione occurred to give 1:1 and 1:2 cycloadducts which further underwent iron-catalyzed annulative diarylation. The thus-formed 1:1 and 1:2 adducts were subjected to radical-mediated oxidation and thermal cycloreversion to furnish one-side and two-side π-extended rubrenes, respectively. These π-extended rubrenes displayed a marked red shift in absorption and emission spectra, clearly showing that the acene π-system of rubrene was extended not only structurally but also electronically. The X-ray crystallographic analysis uncovered interesting packing modes of these π-extended rubrenes. Particularly, two-side π-extended rubrene adopts a brick-wall packing structure with largely overlapping two-dimensional face-to-face π–π interactions. Finally, organic field-effect transistor devices using two-side π-extended rubrene were fabricated, and their carrier mobilities were measured. The observed maximum hole mobility of 1.49 × 10–3 cm2V–1 s–1, which is a comparable value to that of the thin-film transistor using rubrene, clearly shows the potential utility of two-side π-extended rubrene in organic electronics.