

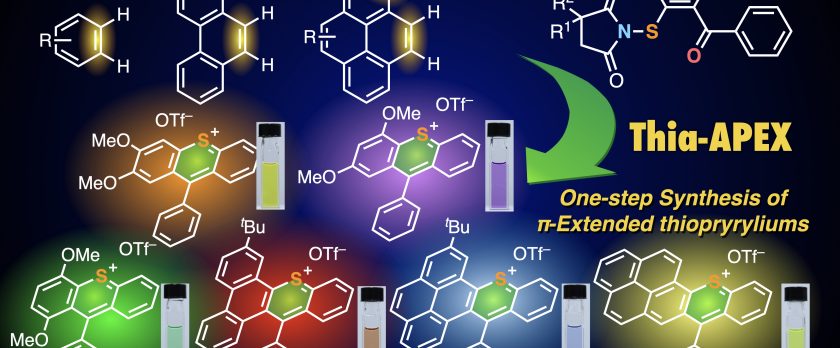
Rapid access to polycyclic thiopyrylium compounds from unfunctionalized aromatics by thia-APEX reaction
Kou. P. Kawahara, Hideto Ito,* Kenichiro Itami*
Chem. Commun. 2023, 59, 1157–1160. DOI: 10.1039/D2CC06706D.
We developed a sulfur-embedding annulative π-extension (thia-APEX) reaction that could construct a sulfur-embedding cationic hexagonal aromatic ring, thiopyrylium, onto unfunctionalized aromatics in one step. The key of thia-APEX is the use of S-imidated ortho-arenoyl arenethiols, and a variety of π-extended thiopyryliums can easily be synthesized. The synthesized thiopyryliums showed diverse absorption and emission properties over the visible light to NIR region, depending on minor structural differences.