

Yasutomo Segawa, Aiko Fukazawa, Sanae Matsuura, Haruka Omachi, Shigehiro Yamaguchi, Stephan Irle, and Kenichiro Itami
Org. Biomol. Chem. 2012, Advance Article. DOI: 10.1039/C2OB25199J
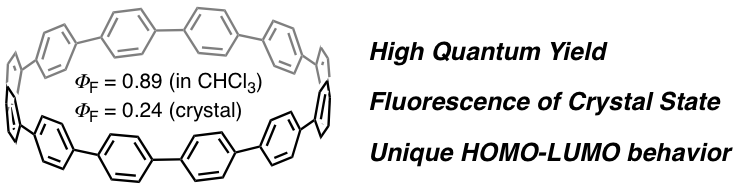
We studied the UV-vis absorption and fluorescence in solution/solid states of [n]cycloparaphenylene ([n]CPP: n = 9, 12, 14, 15, and 16), and conducted theoretical studies to better understand the experimental results. The representative experimental findings include (i) the most intense absorption maxima (λabs1) display remarkably close values (338–339 nm), (ii) the longest-wavelength absorption maxima (λabs2) are blue-shifted with increasing the ring size (395 → 365 nm), (iii) the emission maxima (λem) are blue-shifted with increasing the ring size (494 → 438 nm for longest-wavelength maxima), (iv) the fluorescent quantum yields (ΦF) in solution are high (0.73–0.90), (v) the fluorescence lifetimes (τs) of [9]- and [12]CPP are 10.6 and 2.2 ns, respectively, and (vi) the ΦF values slightly increase in polymer matrix but significantly decrease in the crystalline state. According to TD-DFT calculations, the longest-wavelength absorption (λabs2) corresponds to a forbidden HOMO → LUMO transition and the most intense absorption (λabs1) corresponds to degenerate HOMO − 1 → LUMO and HOMO → LUMO + 1 transitions with high oscillator strength. The interesting and counterintuitive optical properties of CPPs (constant λabs1 and blue shift of λabs2) could be ascribed mainly to the ring-size effect in frontier molecular orbitals (in particular the increase of the HOMO–LUMO gap as the number of benzene rings increases). On the basis of comparative calculations using hypothetical model geometries, we conclude that the unique behavior of HOMO and LUMO of CPPs is due mainly to their lack of a conjugation length dependence in combination with a significant bending effect (particularly to HOMO) and a torsion effect (particularly to LUMO).