

Yasutomo Segawa*, Motonobu Kuwayama, Yuh Hijikata, Masako Fushimi, Taishi Nishihara, Jenny Pirillo, Junya Shirasaki, Natsumi Kubota, Kenichiro Itami*
Science 2019, 365, 272-276. DOI: 10.1126/science.aav5021
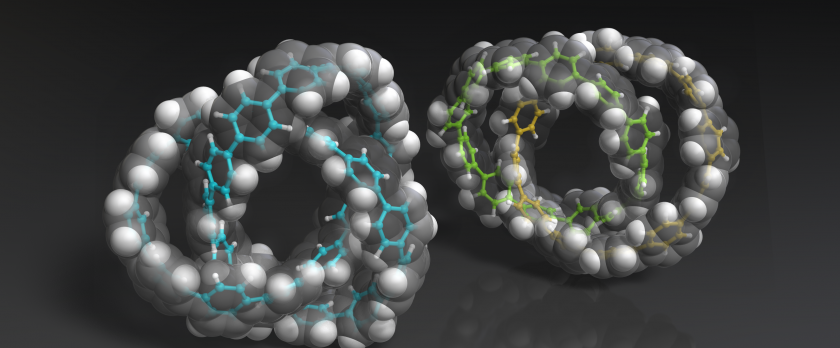
The generation of topologically complex nanocarbons can spur developments in science and technology. However, conventional synthetic routes to interlocked molecules require heteroatoms. We report the synthesis of catenanes and a molecular trefoil knot consisting solely of para-connected benzene rings. Characteristic fluorescence of a heterocatenane associated with fast energy transfer between two rings was observed, and the topological chirality of the all-benzene knot was confirmed by enantiomer separation and circular dichroism spectroscopy. The seemingly rigid all-benzene knot has rapid vortex-like motion in solution even at -95°C, resulting in averaged nuclear magnetic resonance signals for all hydrogen atoms. This interesting dynamic behavior of the knot was theoretically predicted and could stimulate deeper understanding and applications of these previously untapped classes of topological molecular nanocarbons.