

Masakazu Nambo, Atsushi Wakamiya, Shigehiro Yamaguchi and Kenichiro Itami*, J Am. Chem. Soc. 2009, ASAP.
DOI: 10.1021/ja9071173
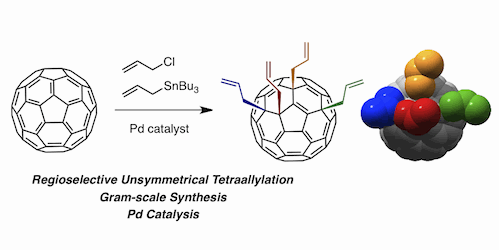
A Pd-catalyzed tetraallylation of C60 that selectively occurs with an unsymmetrical addition pattern has been established. Treatment of C60 with CH2=CHCH2Cl, CH2=CHCH2SnBu3, and PdCl2[P(OPh)3]2 in 1,2-Cl2C6H4 at 50 °C afforded the tetraallylated fullerene in 74% yield with virtually complete regioselectivity. Mechanistic analysis has revealed that (i) tetraallylation proceeds through consecutive nucleophilic/electrophilic allylations [likely via bis(pi-allyl)palladium intermediate], and (ii) both steric (for the first diallylation) and electronic (for the second diallylation) factors are responsible for the high regioselectivity. Moreover, interesting ramifications of the current mechanistic picture include significant opportunities for enantioselective synthesis of tetraallylated fullerene by rendering the final C60-allyl bond-forming event enantioposition-selective with a chiral ligand on Pd. As the four chemically nonequivalent allyl groups should in principle be discriminated in further chemical transformations, a variety of chiral nanoarchitectures would be accessible.