

Anna Junker, Junichiro Yamaguchi, Kenichiro Itami, and Bernhard Wünsch
J. Org. Chem. 2013, , Accepted Manuscript. DOI: 10.1021/jo400692p
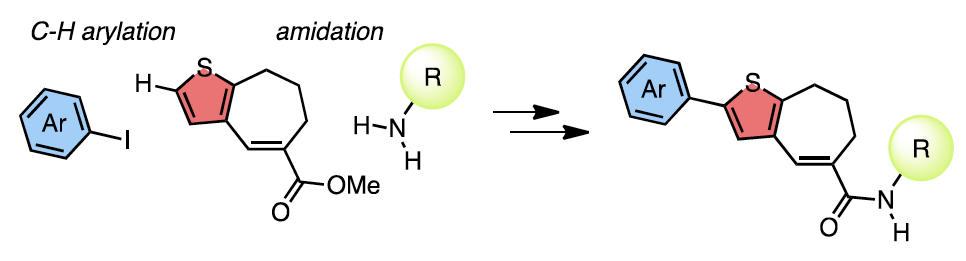
Late-Stage Diversification Strategy
A rapid synthesis of thiophene-based TAK-779 analogues is reported using a late-stage diversification strategy. At the end of the synthesis, the key building block, which was prepared in six steps from thiophene, was arylated regioselectively at the α-position directly with iodoarenes.