

Christina Meyer, Dirk Schepmann, Shuichi Yanagisawa, Junichiro Yamaguchi, Valentina Dal Col, Erik Laurini, Kenichiro Itami, Sabrina Pricl, and Bernhard Wünsch
J. Med. Chem. 2012, ASAP. DOI: 10.1021/jm300894h
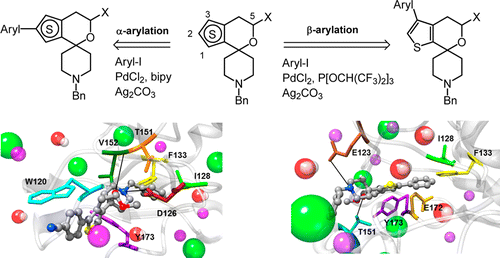
To explore the hydrophobic binding region of the σ1 receptor protein, regioisomeric spirocyclic thiophenes 9–11 were developed as versatile building blocks. Regioselective α- and β-arylation using the catalyst systems PdCl2/bipy/Ag2CO3 and PdCl2/P[OCH(CF3)2]3/Ag2CO3 allowed the introduction of various aryl moieties at different positions in the last step of the synthesis. The increasing σ1 affinity in the order 4 < 5/6 < 7/8 indicates that the positions of the additional aryl moiety and the S atom in the spirocyclic thiophene systems control the σ1 affinity. The main features of the pharmacophore model developed for this class of σ1 ligands are a positive ionizable group, a H-bond acceptor group, two hydrophobic moieties, and one hydrophobic aromatic group. Docking of the ligands into a σ1 3D homology model via molecular mechanics/Poisson–Boltzmann surface area calculations led to a very good correlation between the experimentally determined and estimated free energy of receptor binding. These calculations support the hypothesis of a reverse binding mode of ligands bearing the aryl moiety at the “top” (compounds 2, 3, 7, and 8) and “left” (compounds 4, 5, and 6) positions, respectively.