

Yutaro Saito, Kotono Yamanoue, Yasutomo Segawa,* and Kenichiro Itami*
Chem 2020, DOI: 10.1016/j.chempr.2020.02.004
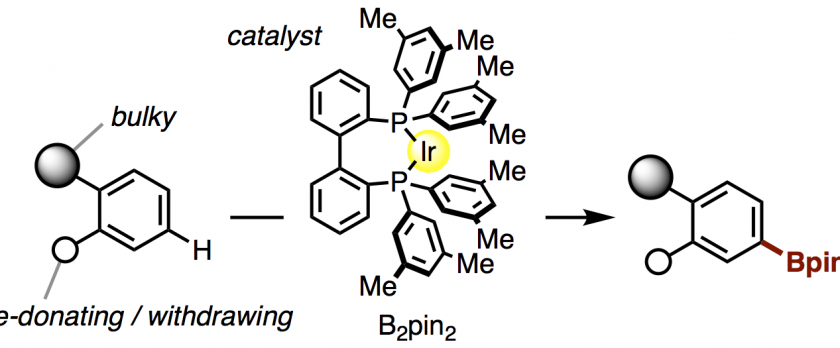
C–H functionalizations of complex molecules such as natural products, pharmaceuticals, and π-conjugated systems are at the heart of constructing and modifying organic molecules, whereby the selectivity and predictability are of the utmost importance. Herein, we report the highly C3-selective C–H borylation of strychnine along with olefin isomerization, catalyzed by an iridium complex with a diphosphine ligand. This method enabled us to rapidly produce 15 strychnine derivatives by using the corresponding C3-borylated and isomerized analog as the common synthetic intermediate. The present catalyst system was also generally effective for the C–H functionalization of unsymmetric 1,2-disubstituted benzene derivatives, including fused π-systems (xanthenes, fluorenes, naphthalenes, and anthracenes) and pharmaceuticals (Nifedipine), in which the C–H positions furthest away from the bulky groups were borylated with high selectivity.