

Kei Muto, Taito Hatakeyama, Junichiro Yamaguchi and Kenichiro Itami,
Chem. Sci. 2015, Advanced Article. DOI: 10.1039/C5SC02942B
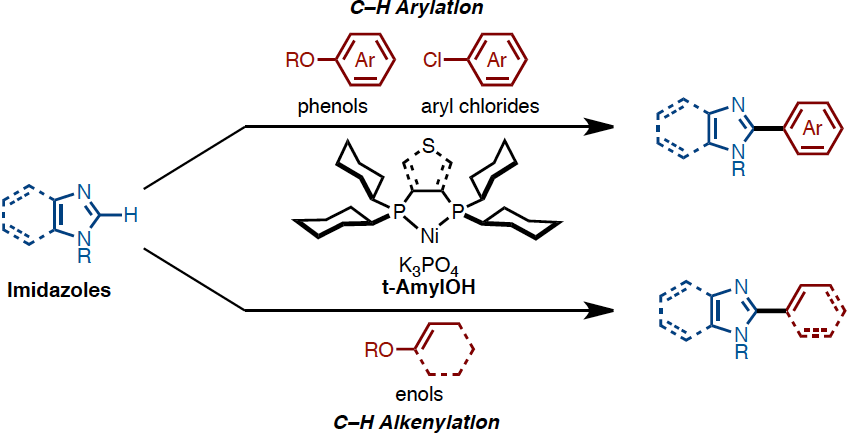
The first nickel-catalyzed C-H arylation and alkenylation of imidazoles with phenol and enol derivatives
Under the influence of Ni(OTf)2/dcype/K3PO4 (dcype: 1,2-bis(dicyclohexylphosphino)ethane) in t-amyl alcohol, imidazoles can undergo C-H arylation with phenol derivatives. The C-H arylation of imidazoles with chloroarenes as well as that of thiazoles and oxazoles with phenol derivatives can also be achieved with this catalytic system. By changing the ligand to dcypt (3,4-bis(dicyclohexylphosphino)thiophene), enol derivatives could also be employed as coupling partners achieving the C-H alkenylation of imidazole as well as thiazoles and oxazoles. Thus, a range of C2-arylated and alkenylated azoles can be synthesized using this newly developed nickel-based catalytic systems. The key to success of C-H coupling of imidazoles is the use of tertiary alcohol as solvent. This also allows using air-stable nickel(II) salt as a catalyst precursor.