

Kei Muto, Junichiro Yamaguchi, and Kenichiro Itami
J. Am. Chem. Soc. 2011, ASAP. DOI: 10.1021/ja210249h
Highlighted in Newspapers (Yomiuri, Chu-nichi, The Chemical Daily)
Pave new way toward ideal cross coupling
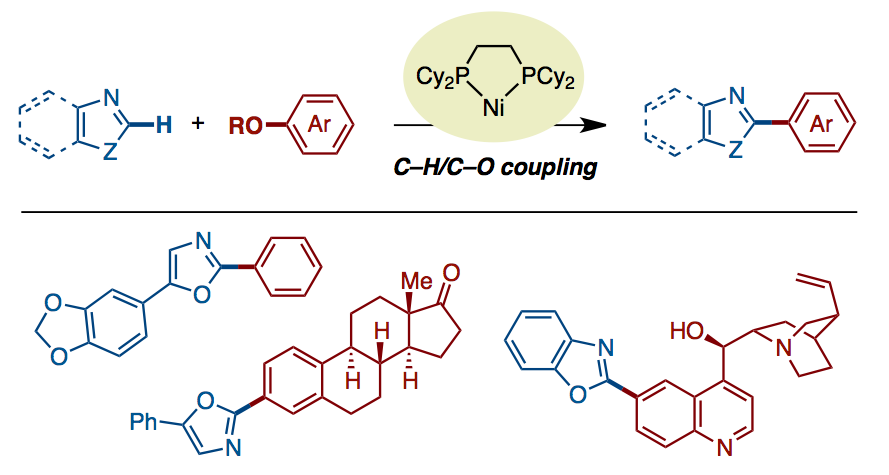
The first nickel-catalyzed C–H bond arylation of azoles with phenol derivatives have been developed. The new Ni(cod)2/dcype catalytic system is active for the coupling of various phenol derivatives such as esters, carbamates, carbonates, sulfamates, triflates, tosylates, and mesylates. By using the C–H/C–O biaryl coupling, a series of privileged 2-arylazoles including biologically active alkaloids were synthesized. Moreover, we demonstrated the utility of present reaction for functionalizing estrone and quinine.
[Nagoya University Press Release]