Satoshi Tani, Takahiro N. Uehara, Junichiro Yamaguchi and Kenichiro Itami, Chem. Sci. 2013, ASAP. DOI: 10.1039/C3SC52199K
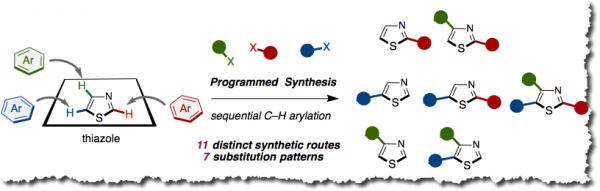
Programmed Synthesis Thiazoles attached with aryl or heteroaryl groups (arylthiazoles) represent privileged structural motifs that are frequently utilized in functional organic materials including organic electroluminescent devices, as well as in bioactive compounds and pharmaceuticals. Thus, an efficient synthetic methodology that allows cost- and step-efficient production of arylthiazoles is in high demand. Moreover, in a situation where the structure-property relationships are not predictable, a uniform and programmable design that allows the synthesis of all possible arylthiazoles would help accelerate identifying and optimizing the “functional” arylthiazole structures. Herein we report a programmed synthesis of all substitution patterns of arylthiazoles (2-aryl, 4-aryl, 5-aryl, 2,4-diaryl, 2,5-diaryl, 4,5-diaryl, and 2,4,5-triaryl) via sequential C-H coupling catalyzed by palladium or nickel. Noteworthy features of our method are: (i) the synthetic expediency in introducing aryl groups by step-economical, direct C-H arylation, (ii) all aryl groups assembled stem from readily available aryl halides or arylboronic acids, (iii) the aryl-group-installation at the desired position can be achieved by the choice of catalytic systems, and (iv) the possibility of synthesizing all substitution patterns of arylthiazoles by 11 distinct pathways. Using this protocol, we have accomplished the synthesis of over 150 different arylthiazoles, among which exists fatostatin, known as a SREBP inhibitor. This method can also be applied to a gram-scale synthesis of triarylthiazoles.