

Kei Muto, Junichiro Yamaguchi, Aiwen Lei, and Kenichiro Itami J. Am. Chem. Soc. 2013, ASAP DOI: 10.1021/ja409803x
Isolation, Structure, and Reactivity of an Ar–Ni–OR!
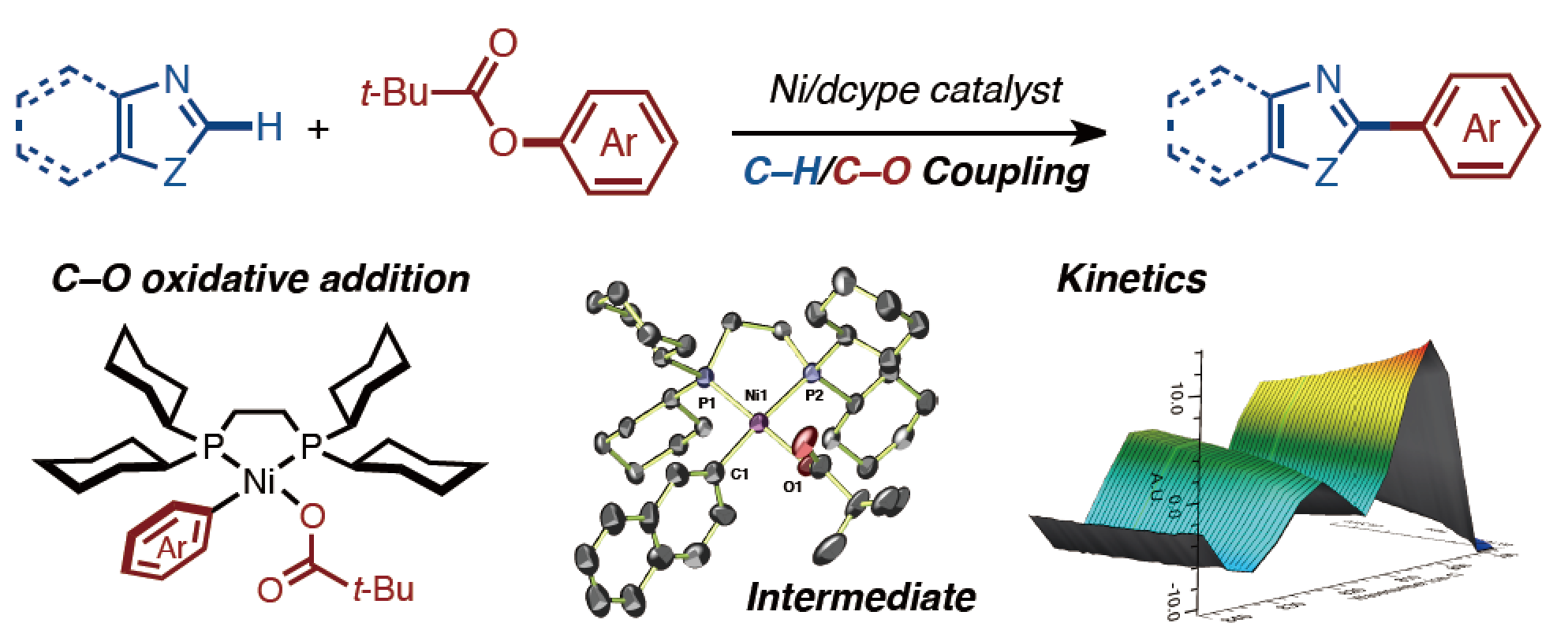
This paper describes mechanistic studies of a C–H/C–O biaryl coupling of 1,3-azoles and aryl pivalates catalyzed by Ni(cod)2/dcype. This study not only supports a catalytic cycle consisting of C–O oxidative addition, C–H nickelation, and reductive elimination, but also provides insight into the dramatic ligand effect in C–H/C–O coupling. We have achieved the first synthesis, isolation and structure elucidation of an arylnickel(II) pivalate, which is an intermediate in the catalytic cycle after oxidative addition of a C–O bond. Furthermore, kinetic studies and kinetic isotope effect investigations reveal that the C–H nickelation is the turnover-limiting step in the catalytic cycle.