

Kazuma Amaike,Kenichiro Itami, and Junichiro Yamaguchi
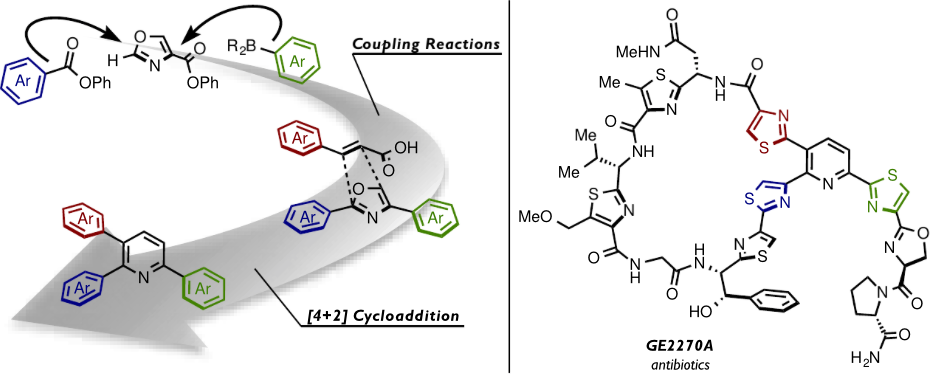
We have described a C-H arylation/ring transformation strategy for the synthesis of triarylpyridines, which form the core structure of thiopeptide antibiotics. This synthetic method readily provided 2,3,6-triarylpyridines in a regioselective manner by a two-phase approach: C-H arylation (a nickel-catalyzed decarbonylative Suzuki-Miyaura cross-coupling and decarbonylative C-H coupling for the synthesis of 2,4-diaryloxazoles) and ring transformation ([4+2] cycloaddition of 2,4-diaryloxazoles with (hetero)arylacrylic acids). To showcase these methods, we have accomplished the formal synthesis of thiopeptide antibiotics GE2270s and amythiamicins.