

Atsushi D. Yamaguchi, Kathryn M. Chepiga, Junichiro Yamaguchi, Kenichiro Itami, and Huw M. L. Davies,
J. Am. Chem. Soc. 2015, ASAP. DOI: 10.1021/ja512059d
Natural Products Synthesis Using C–H functionalizations
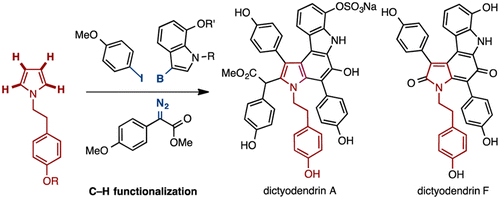
Syntheses of dictyodendrins A and F have been achieved using a sequential C–H functionalization strategy. The N-alkylpyrrole core is fully functionalized by means of a rhodium(I)-catalyzed C–H arylation at the C3-position, a rhodium(II)-catalyzed double C–H insertion at the C2- and C5-positions, and a Suzuki–Miyaura cross-coupling reaction at the C4-position. The syntheses of dictyodendrins A and F were completed by formal 6π-electrocyclization to generate the pyrrolo[2,3-c]carbazole core of the natural products.