

Hua Zhang, Shinya Hagihara, and Kenichiro Itami
Chem. Eur. J. 2015, 21, 16796-16800. DOI: 10.1002/chem.201503596
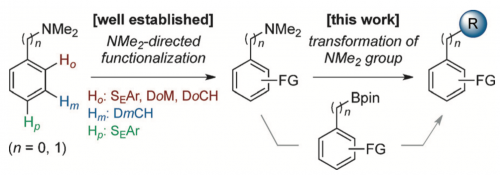
The dimethylamino (Me2N) group is arguably the most versatile functional group capable of highly efficient and site-selective directed aromatic functionalizations at the ortho-, meta-, and para-positions depending on reaction conditions. While the repertoire of Me2N-directed reactions is growing at a rapid pace, the lack of a general method to transform this group to other functionalities hampers its wider application in organic synthesis. Here we report nickel-catalyzed CN borylations of aryl- and benzyl-dimethylamines that permit the conversion of a huge library of largely underutilized Me2N-containing organic molecules into various functional molecules by taking advantage of the wealth of existing CB functionalization methods.