

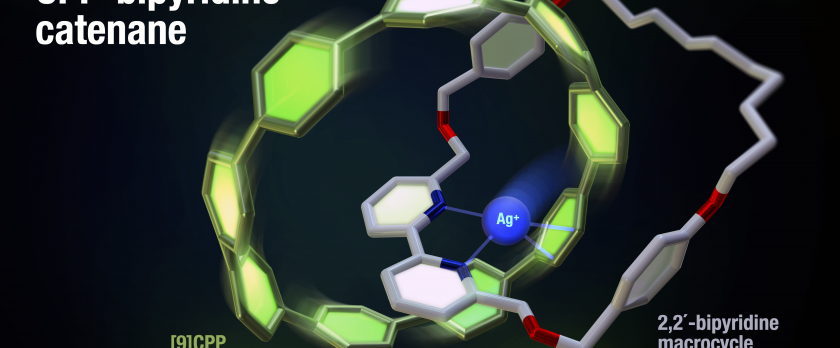
The active metal template (AMT) strategy is a powerful tool for the formation of mechanically interlocked molecules (MIMs) such as rotaxanes and catenanes, allowing the synthesis of a variety of MIMs, including π-conjugated and multicomponent macrocycles. Cycloparaphenylene (CPP) is an emerging molecule characterized by its cyclic π-conjugated structure and unique properties. Therefore, diverse modifications of CPPs are expected for its wide application. However, most CPP modifications require early-stage functionalization and the direct modification of CPPs is very limited. Herein, we report the synthesis of a catenane consisting of [9]CPP and a 2,2´-bipyridine macrocycle as a new CPP analogue that contains a reliable synthetic scaffold enabling diverse and concise post-modification. Following the AMT strategy, [9]CPP–bipyridine catenane was successfully synthesized through Ni-mediated aryl–aryl coupling. Catalytic C–H borylation/cross-coupling and metal complexation at bipyridine macrocycle moiety, an effective post-functionalization method, were also demonstrated with [9]CPP–bipyridine catenane. Single crystal X-ray structural analysis revealed that the [9]CPP–bipyridine catenane forms a tridentated complex with an Ag ion inside the CPP ring. This interaction significantly enhances phosphorescence lifetime through improved intermolecular interactions. |