

Christina Meyer, Dirk Schepmann, Shuichi Yanagisawa, Junichiro Yamaguchi, Bernhard Wünsch, and Kenichiro Itami
Eur. J. Org. Chem. 2012, Early View. DOI: 10.1002/ejoc.201200837
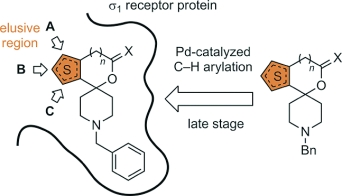
Direct C–H bond arylation in the α- and β-positions of spirocyclic thiophenes containing various functional groups (amine, ether, acetal, lactone) was accomplished. Selective phenylation in the α-position of the thiophene ring was achieved by using the catalytic system PdCl2/bipy/Ag2CO3. The introduction of phenyl moieties to the β-position was performed with the catalytic system PdCl2/P[OCH(CF3)2]3/Ag2CO3. Even the five-membered lactone 10 with an electron-withdrawing carbonyl moiety directly attached to the thiophene ring was arylated. Spirocyclic thiophenes substituted with a phenyl moiety in position A (top position) or B (left position) display low nanomolar σ1 affinities (e.g., 4a: Ki = 1.6 nM; 5a: Ki = 2.4 nM), indicating an additional hydrophobic pocket on the complementary σ1 receptor protein. A phenyl moiety in position C (at the bottom position) is not tolerated by the σ1 receptor (e.g., 12: Ki = 483 nM). However, an additional phenyl moiety in position A is able to compensate at least partially the unfavorable effects of the phenyl moiety in position C.