

Masakazu Nambo, Atsushi Wakamiya, and Kenichiro Itami
Chem. Sci. 2012, Advance Article. DOI: 10.1039/C2SC21126B
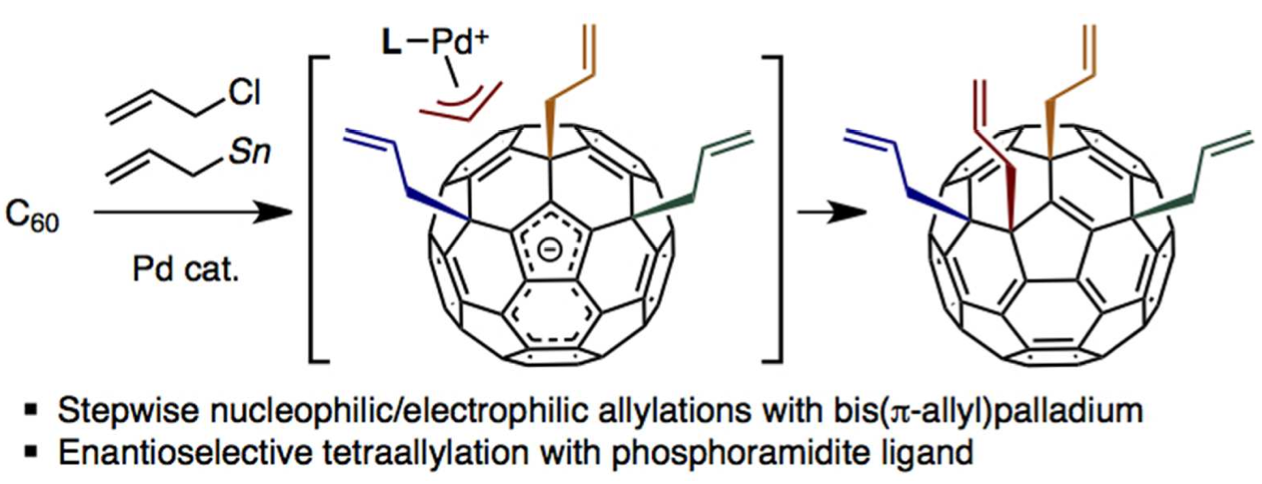
We have established a unique Pd-catalyzed tetraallylation of C60 with allyl chloride and allylstannane that likely proceeds by the action of amphiphilic bis(π-allyl)palladium. Mechanistic analysis has revealed that both steric (for the first diallylation) and electronic (for the second diallylation) factors are responsible for high regioselectivity. The ring-closing metathesis reaction and hydrogenation of tetraallylated product took place in the presence of Ru and Rh catalysts. Moreover, we found that chiral phosphoramidites are effective chiral ligands for the enantioselective tetraallylation of C60. Pronounced enantioselectivity up to 88% ee was realized in the production of tetraallylated C60.