“Synthesis, properties, and material hybridization of bare aromatic polymers enabled by dendrimer support”
Shusei Fujiki, Kazuma Amaike, Akiko Yagi,* and Kenichiro Itami*
Nature Communications 2022, accepted. DOI: 10.1038/s41467-022-33100-7
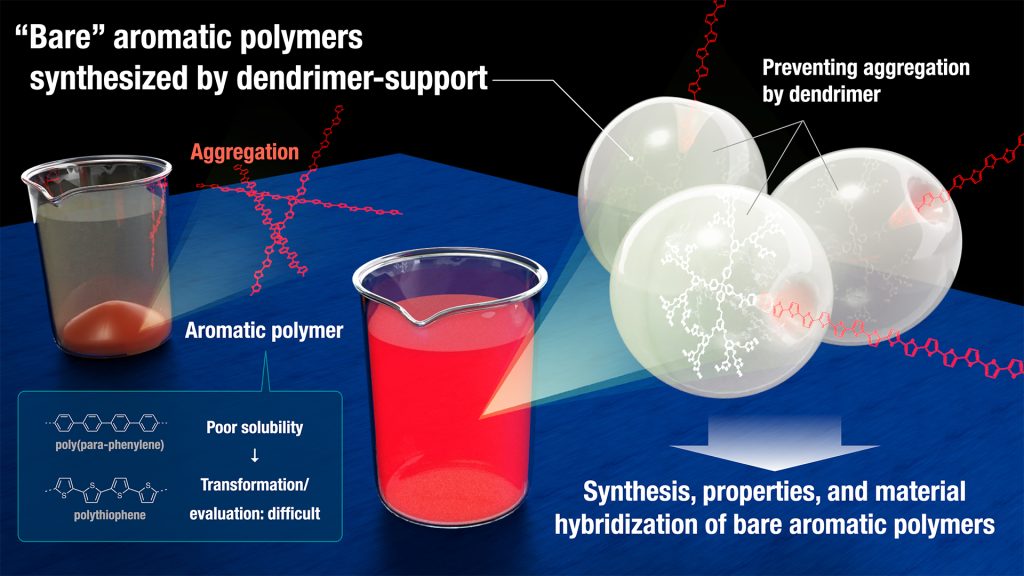
Aromatic polymers are one of the central and essential materials supporting our daily life and serve as the first-choice platform for next-generation materials due to their distinct optical, electronic, and mechanical properties as well as their biocompatibility. In particular, poly(para-phenylene)s (PPPs) and polythiophenes (PTs) have received a tremendous amount of interest due to their high performance as conducting and luminescent materials. As their properties critically depend on structural factors, chemists have devoted decades of efforts in the precise synthesis of aromatic polymers. However, due to strong intermolecular p-p interactions, the parent PPPs and PTs (without any substituents) are insoluble to solvents; these molecules are already barely soluble at six monomer units (e.g., the solubility of terphenylene; 8.5 g/L vs sexiphenylene; < 0.01 g/L). Because of this solubility problem, the direct synthesis, property analysis, and material manipulation/transfer of long bare aromatic polymers have remained elusive. While several alternative methods using well-designed soluble precursors or surface-assisted synthesis have been developed for PPP synthesis, bare PPPs can only be obtained as insoluble aggregates which are hard to use for any analysis or application.
We have developed a general method to handle main chain-unsubstituted “bare” aromatic polymers in solution, which is otherwise difficult because of poor solubility. The key is hanging the aromatic polymers to a dendrimer which is designed by ourselves, preventing aggregation owing to its giant structure.
*The picture is created by Dr. Issey Takahashi, ITbM, Nagoya University.