

Kazuya Kawai, Kenta Kato, Lingqing Peng, Yasutomo Segawa,* Lawrence T. Scott, and Kenichiro Itami*
Org. Lett. 2018, DOI: 10.1021/acs.orglett.8b00477
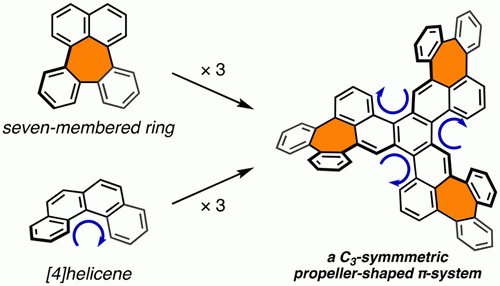 The synthesis and structure of a C3-symmetric propeller-shaped polycyclic aromatic hydrocarbon that bears three seven-membered rings is reported. The synthesis was accomplished in three steps from benzo[c]naphtho[2,1-p]chrysene, including a Pd-catalyzed intramolecular C–H arylation for the formation of the seven-membered rings. The combination of the helicities (P/M) of the three seven-membered-ring moieties and three [4]helicene moieties affords 24 possible conformers, and two relatively stable conformations were observed by 1H NMR spectroscopy.
The synthesis and structure of a C3-symmetric propeller-shaped polycyclic aromatic hydrocarbon that bears three seven-membered rings is reported. The synthesis was accomplished in three steps from benzo[c]naphtho[2,1-p]chrysene, including a Pd-catalyzed intramolecular C–H arylation for the formation of the seven-membered rings. The combination of the helicities (P/M) of the three seven-membered-ring moieties and three [4]helicene moieties affords 24 possible conformers, and two relatively stable conformations were observed by 1H NMR spectroscopy.