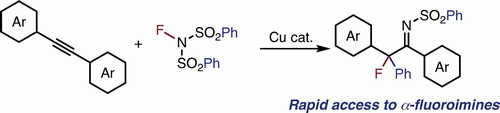
Shu Jan Yip, Tetsushi Yoshidomi, Kei Murakami, Kenichiro Itami*
Chem. Lett. 2018, 47, 329–331. DOI: 10.1246/cl.171097
Difunctionalization of unsaturated bonds with fluorinating reagents has been regarded as an efficient route to vic- difunctional organic fluorides. Here we report a copper- catalyzed synthesis of α-fluoroimines from diarylacetylenes and N-fluorobenzenesulfonimide. Mechanistically, the reaction initiates from the generation of imidyl radical, which reacts with alkyne to give the product. The resulting α-fluoroimine can easily be transformed into other organic fluorides such as fluoroketone and fluoroamines.