

Taishi Nishihara, Yasutomo Segawa, Kenichiro Itami, and Yoshihiko Kanemitsu
J. Phys. Chem. Lett. ASAP. DOI: 10.1021/jz3014826
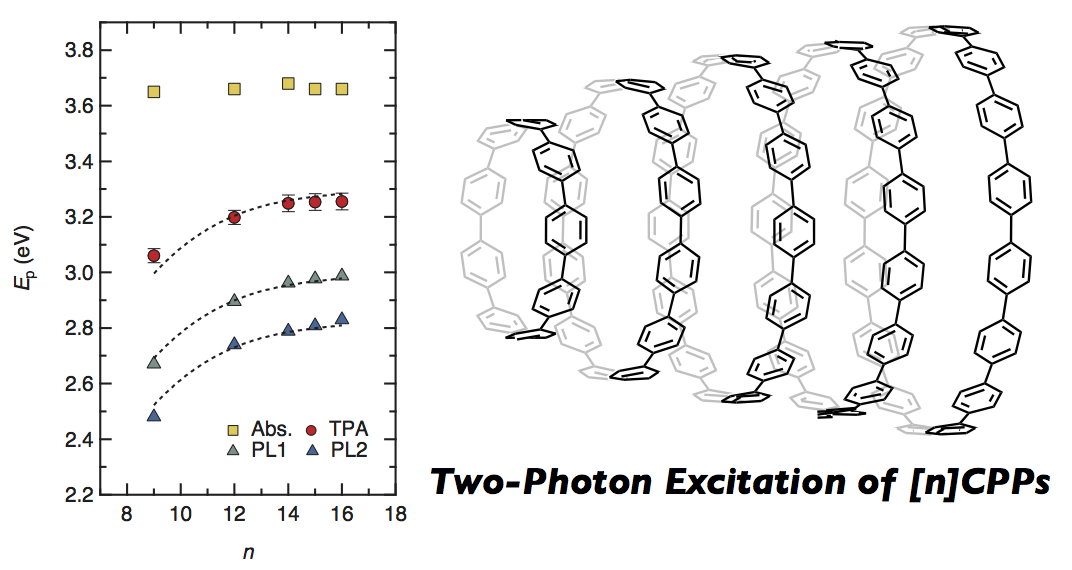
Hoop-shaped conjugated molecules, cycloparaphenylenes (CPPs), are simple strings of benzene rings with para linkages that have an ideal quasi-one-dimensional structure without edges. Here, we report optical properties of [n]CPPs (n = 9, 12, 14, 15, 16) clarified by one- and two-photon excitation spectroscopy. We showed that in this system the lowest unoccupied molecular orbital (LUMO) state has the same symmetry as the highest occupied molecular orbital (HOMO) state, and determined the transition energy of the optically forbidden HOMO–LUMO gap. It is found that the ring-length dependence of the HOMO–LUMO transition energy is identical to that of the photoluminescence (PL) energy, and that phonon-assisted transition causes efficient PL.