

Shuya Yamada, Kei Murakami, and Kenichiro Itami
Org. Lett. 2016, 18, 2415–2418. doi: 10.1021/acs.orglett.6b00932
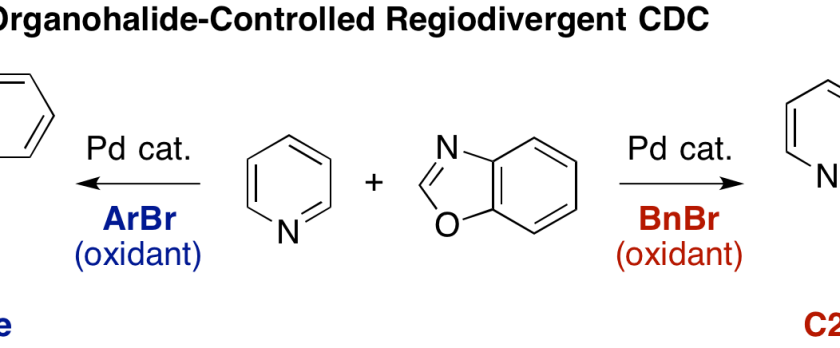
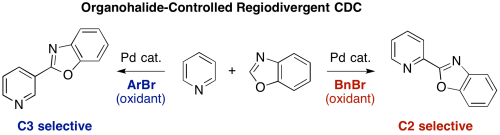
Pyridine is a scaffold that is ubiquitously found in various natural products, pharmaceuticals, agrochemicals, and materials. Herein, we have developed regioselective C–H/C–H oxidative coupling of pyridine and oxazole under palladium catalysis. The regioselectivity can be controlled by the choice of oxidants. Employment of bromomesitylene afforded the C3-azolylpyridines whereas benzyl bromide worked as an efficient oxidant to give the C2-azolylpridines.
Keywords: Palladium Catalyst; Cross-dehydrogenative Oxidative Coupling; C–H Functionalization; Pyridine