

Takise, R.; Isshiki, R. Muto K.; Itami, K.; Yamaguchi, J.
J. Am. Chem. Soc. 2017, Just Accepted Manuscripts. DOI: 10.1021/jacs.7b00049
Abstract
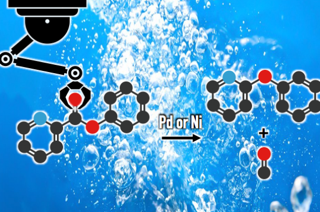
Since diaryl ethers are present as an important motif in pharmaceuticals and natural products, extensive studies for the development of novel methods have been conducted. A conventional method for the construction of the diaryl ether moiety is the intermolecular cross-coupling reaction of aryl halides and phenols with a copper or palladium catalyst. We developed a catalytic decarbonylative etherification of aromatic esters using a palladium or nickel catalyst with our enabling diphosphine ligand to give the corresponding diaryl ethers. The present reaction can be conducted on gram scale in excellent yield. This reaction not only functions in an intramolecular setting, but also allows for a cross-etherification using other phenols.
Comments from Mr. Ryosuke Takise (sorry, this contents is only Japanese)
もともとは違う反応を追っていて偶然見つけたこのFromエステルToエーテル反応。一酸化炭素が抜けるだけという非常にシンプルな反応です。基質適用範囲にまだ制限がありますが、分子変換としては非常に興味深く、個人的にもお気に入りの反応です。パラジウムを担当してくれた一色遼太くんと一緒に研究ができたのも良い経験になりました。本反応に用いられるdcyptという配位子は近日中に市販化が可能になりますので、ぜひ利用してみてください。たきしでし!