

Tetsushi Yoshidomi, Tomohiro Fukushima, Kenichiro Itami, Yasutomo Segawa
Chem. Lett. 2017, DOI: 10.1246/cl.170091
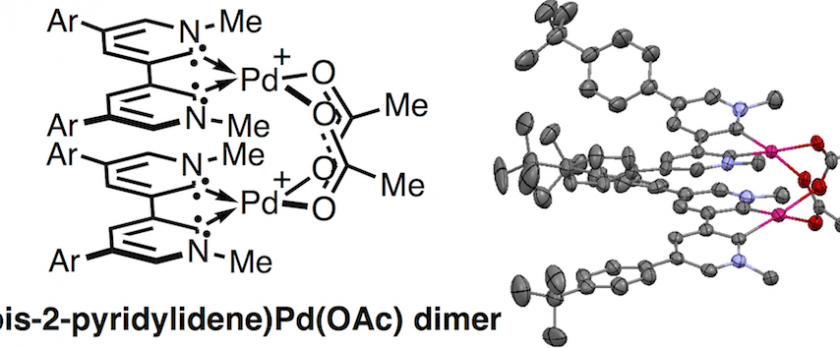
A bimetallic acetate-bridged (bis-2-pyridylidene)Pd(II) complex was synthesized and its oxidation behavior was investigated by cyclic voltammetry. The density functional theory calculation indicated that bis-2-pyridylidene is a potentially useful ligand to stabilize bimetallic Pd(III) species owing to its strong electron donation ability and high planarity. The di-tert-butylphenyl substituted (bis-2-pyridylidene)Pd(II) carbonate complex was synthesized via sequential C–H borylation and Suzuki–Miyaura cross-coupling reactions, and the carbonate complex was successfully converted into an acetate-bridged bimetallic complex. The structural parameters and oxidation potentials of the acetate-bridged (bis-2-pyridylidene)Pd(II) complex indicated the strong electron donation ability of the bis-2-pyridylidene ligand compared with neutral bidentate ligands. Despite several attempts, (bis-2-pyridylidene)Pd(III) complexes could not be isolated because of their instability.