

Kyohei Ozaki, Wataru Matsuoka, Hideto Ito, Kenichiro Itami
Org. Lett., 2017, 19, 1930–1933. DOI: 10.1021/acs.orglett.7b00684
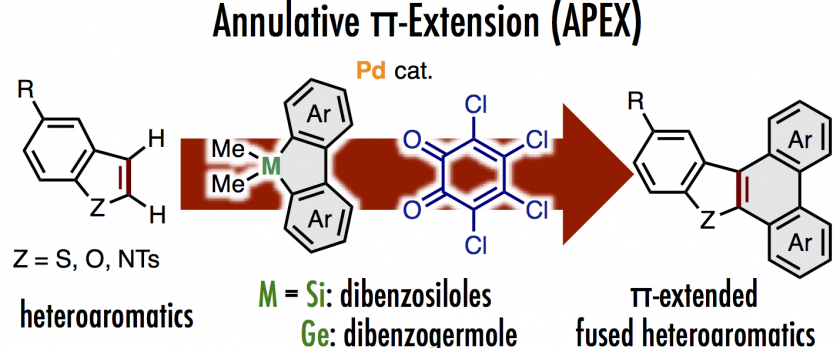
Annulative π-extension (APEX) reactions of heteroarenes are described herein. A catalytic system comprising a cationic palladium species and o-chloranil using dimethyldibenzosiloles as π-extending agents enabled the extension of the π-system of benzo[b]thiophenes. π-Extended dibenzofurans and carbazoles could also be obtained from benzofuran and N-tosylindole, respectively, with dimethyldibenzogermole as a germanium-based π-extending agent. Mechanistic investigations indicated two possible reaction pathways involving carbopalladation-based double C–H arylation of benzothiophene or formal cycloaddition/oxidation cascades.
This is a final work by Dr. Ozaki who was Ph.D student in our laboratory until 2016, and also the second paper for Mr. Matsuoka (Mc.student). Congratulations!!