

Kou P. Kawahara, Wataru Matsuoka, Hideto Ito,* Kenichiro Itami*
Angew. Chem. Int. Ed. 2020, 59, 6383-6388. DOI: 10.1002/anie.201913394
Abstract
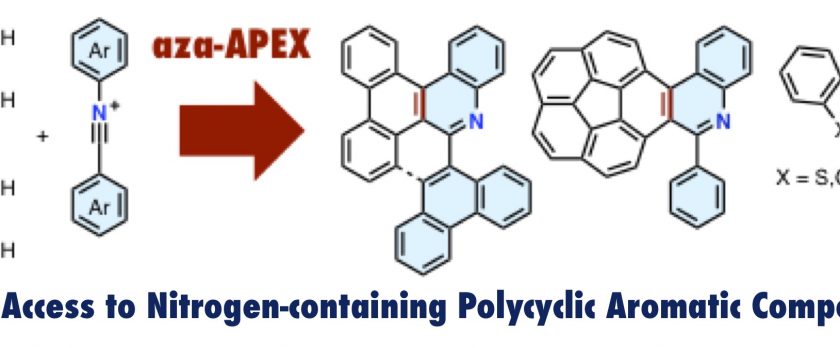
Nitrogen‐containing polycyclic aromatic compounds (N‐PACs) are an important class of compounds in materials science. Reported here is a new aza‐annulative π‐extension (aza‐APEX) reaction that allows rapid access to a range of N‐PACs in 11–84 % yields from readily available unfunctionalized aromatics and imidoyl chlorides. In the presence of silver hexafluorophosphate, arenes and imidoyl chlorides couple in a regioselective fashion. The follow‐up oxidative treatment with p ‐chloranil affords structurally diverse N‐PACs, which are very difficult to synthesize. DFT calculations reveal that the aza‐APEX reaction proceeds through the formal [4+2] cycloaddition of an arene and an in situ generated diarylnitrilium salt, with sequential aromatizations having relatively low activation energies. Transformation of N‐PACs into nitrogen‐doped nanographenes and their photophysical properties are also described.