

Lilia Lohrey, Takahiro N. Uehara, Satoshi Tani, Junichiro Yamaguchi, Hans-Ulrich Humpf and Kenichiro Itami
Eur. J. Org. Chem. 2014, Early View. DOI: 10.1002/ejoc.201402129
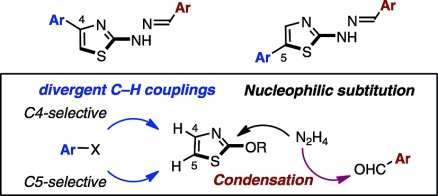
Life-threatening infections caused by bacteria that have developed resistance to common antibiotics, such as methicillin-resistantStaphylococcus aureus (MRSA), have become a serious problem in hospitals and other areas all over the world. Thus, the development of an effective class of antibiotics against these bacteria is an urgent subject. Herein, we report a step-economical and diversity-oriented synthesis of a series of 2-arylidenehydrazinyl-4-arylthiazole and 2-arylidenehydrazinyl-5-arylthiazole analogues that utilizes C–H coupling methodologies. A library of 54 new congeners were synthesized and tested for their biological potential. Moreover, new knowledge regarding the structure–activity relationships (SARs) of these heterobiaryl compounds was collected.