

Shin Suzuki, Kenichiro Itami,* Junichiro Yamaguchi*
Angew. Chem., Int. Ed. 2017, Accepted article. DOI: 10.1002/anie.201709332
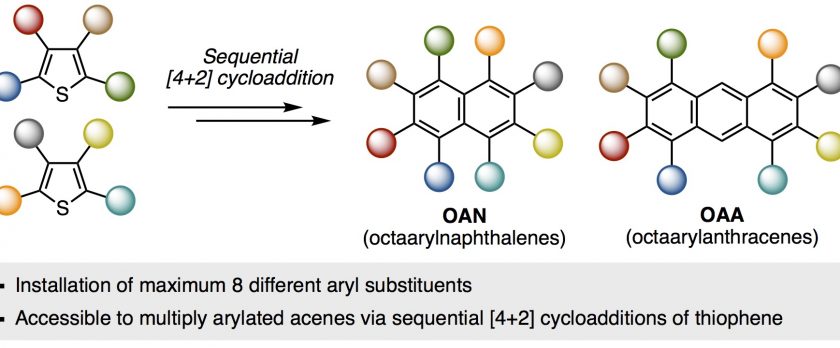
A synthesis of multiply arylated naphthalenes and anthracenes with eight different substituents has been accomplished. The key intermediate is a tetraarylthiophene S-oxides, which is synthesized by a protocol involving sequential C–H arylation and cross-coupling from 3-methoxythiophene, followed by oxidation of the sulfur atom. The resulting tetraarylthiophene S-oxide can be converted to a tetraaryl benzynes or naphthalynes, then merged via a [4+2] cycloaddition reaction with another tetraarylthiophene S-oxide, resulting in the programmed synthesis of octaarylnaphthalene and octaarylanthracene.