

Keishu Okada, Akiko Yagi, Yasutomo Segawa, and Kenichiro Itami
Chem. Sci. 2016, Accepted Manuscript, DOI: 10.1039/C6SC04048A
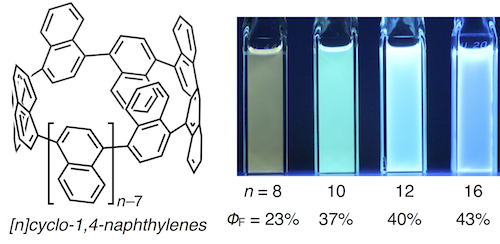
The synthesis and properties of various [n]cyclo-1,4-naphthylenes ([n]CNs, n = 8, 10, 12, and 16) are described. Initially, extended L-shaped units, which could be converted into quater- or quinquenaphthylenes were prepared. Nickel- or palladium-mediated couplings of these extended L-shaped units, followed by reductive aromatization of the coupling products afforded [8]-, [10]-, [12]-, and [16]CNs. The size-dependent photophysical properties of these CNs were confirmed by measuring their UV-vis absorption and fluorescence spectra. The theoretical studies supported substantial effects of the number of naphthalene rings on the structural and photophysical properties of these CNs. A kinetic study on the thermal conversion of the Cs-symmetric conformer of [10]CN into its most stable D5d-symmetric conformer indicated that ring strain affects the rotation barrier of the naphthalene rings in [10]CN.