

Yuuki Ishii, Yusuke Nakanishi, Haruka Omachi, Sanae Matsuura, Katsuma Matsui, Hisanori Shinohara, Yasutomo Segawa, and Kenichiro Itami
Chem. Sci. 2012, Accepted Manuscript. DOI: 10.1039/C2SC20343J
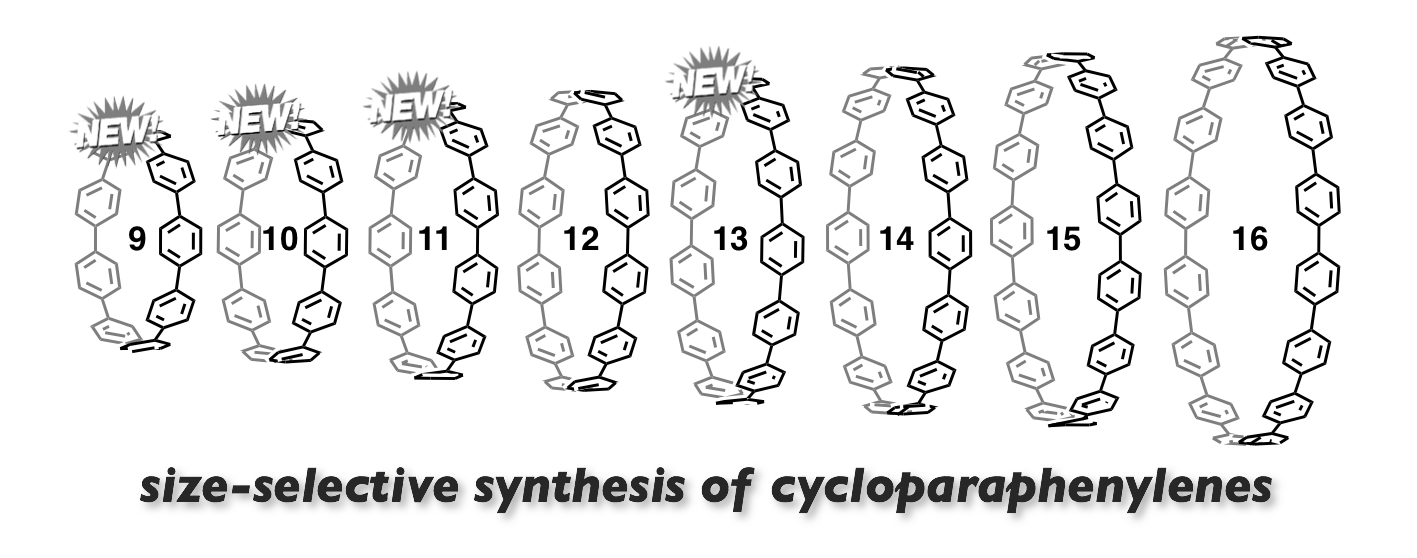
We have achieved the first size-selective synthesis of [9]-[11] and [13]cycloparaphenylenes (CPP) by strategically utilizing cis-1,4-diphenylcyclohexane-1,4-diyl as the key terphenyl-convertible L-shaped unit. To access the designed triangular or rectangular macrocyclic precursors, we utilized palladium-catalysed C-B/C-Br cross-coupling (Suzuki-Miyaura coupling) and/or nickel-mediated C-Br/C-Br coupling. We also established step-economical routes to [14] and [16]CPP using nickel-mediated C-Br/C-r coupling. The final aromatization steps toward CPPs were accomplished with NaHSO4. Thus, combined with our previous size-selective synthesis of [12] and [14]-[16]CPP, we completed our size-selective synthesis of [9]-[16]CPP. The successful size-selective syntheses of [n]CPPs speaks well for the flexibility and reliability of our strategy using a cyclohexane ring.