

Kakishi Uno, Taeko Sasaki, Nagisa Sugimoto, Hideto Ito, Taishi Nishihara, Shinya Hagihara, Tetsuya Higashiyama, Narie Sasaki, Yoshikatsu Sato and Kenichiro Itami
Chem. Asian J. 2017, 12, 233–238. DOI: 10.1002/asia.201601430
Highlighted in chemistry views
Most accessed article in January 2017 (First Place)
Most accessed article in February 2017 (Third Place)
Most accessed article between March 2016 to February 2017 (22nd Place)
Most accessed article between May 2016 to April 2017 (11th place)
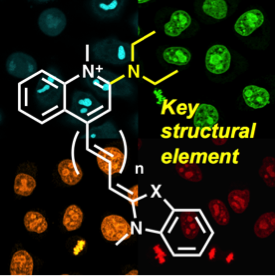
Unsymmetrical cyanine dyes such as thiazole orange are useful for detecting nucleic acids with fluorescence because they dramatically enhance the fluorescence upon binding to nucleic acids. In this paper, we synthesized a series of unsymmetrical cyanine dyes and evaluated their fluorescence properties. A systematic structure-property relationship study has uncovered that the dialkylamino group at the 2-position of quinoline in a series of unsymmetrical cyanine dyes plays a critical role in the fluorescence enhancement. Four newly designed unsymmetrical cyanine dyes showed negligible intrinsic fluorescence in a free-state and strong fluorescence upon binding to double-stranded DNA (dsDNA) with quantum yield 0.53-0.90, which is 2-3 times higher than the previous unsymmetrical cyanine dyes. Detailed analysis of fluorescence lifetime revealed that dialkylamino group at the 2-position of quinoline suppressed non-radiative decay in favor of increasing fluorescence quantum yield. Moreover, these newly developed dyes were able to specifically stain the nucleus in fixed HeLa cells with a confocal laser-scanning microscope.