

Ryosuke Takise, Kenichiro Itami and Junichiro Yamaguchi
Org. Lett. 2016, ASAP. DOI: 10.1021/acs.orglett.6b02265
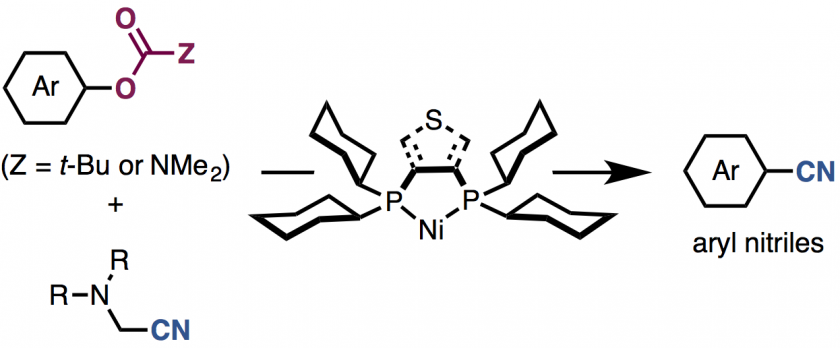
Generation of useful arylnitrile structures from simple aromatic feedstock chemicals represents a fundamentally important reaction in chemical synthesis. The fi rst nickel-catalyzed cya nation of phenol derivatives with metal-free cyanating agents, aminoacetonitriles, is described. A nickel-based catalytic system consisting of a unique diphosphine ligand such as dcype or dcypt enables the cyanation of versatile phenol derivatives such as aryl carbamates and aryl pivalates. The use of aminoacetonitriles as a cyanating agent leads to an environmentally and easy-to-use method for arylnitrile synthesis.