

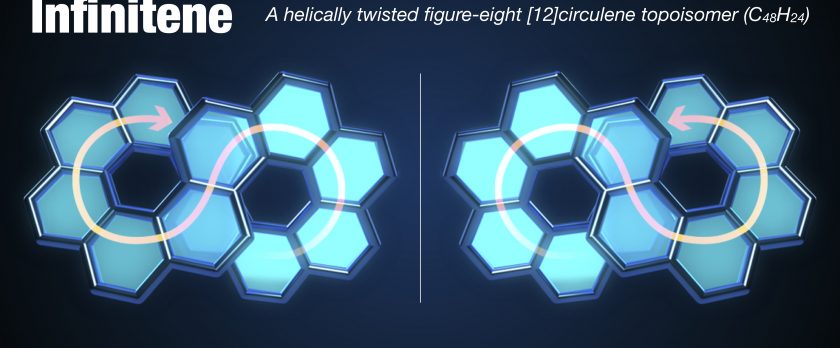
Infinitene: A Helically Twisted Figure-Eight [12]Circulene Topoisomer
Maciej Krzeszewski, Hideto Ito,* Kenichiro Itami*
Submitted. ChemRxiv DOI: 10.33774/chemrxiv-2021-pcwcc [link] [detail]
J. Am. Chem. Soc. 2022, 144, 862–871. DOI: 10.1021/jacs.1c10807
Highlighted in Chemistry Views [link], C&En News [link]
Selected as “The Molecules of The Year 2021” by C&En News, ACS [link] [detail]
Selected as a Supplementary Cover Picture in vol 140 issue 2.
New forms of molecular nanocarbons particularly looped polyarenes adopting various topologies contribute to the fundamental science and practical applications. Here we report on the synthesis of an infinity-shaped polycyclic aromatic hydrocarbon, infinitene 1 (cyclo[c.c.c.c.c.c.e.e.e.e.e.e]dodecakisbenzene) comprising consecutively fused 12-benzene rings forming an enclosed loop with a strain energy of 60.2 kcal·mol-1. Infinitene 1 represents a topoisomer of still-hypothetical [12]circulene, and its scaffold can be formally visualized as the outcome of the “stitching” of two homochiral [6]helicene subunits by their both ends. The synthetic strategy encompasses transformation of a rationally designed dithiacyclophane to cyclophadiene through the Stevens rearrangement and pyrolysis of the corresponding S,S′-bis(oxide) followed by the UV-light mediated twofold photocyclization. The structure of infinitene 1 is a unique hybrid of helicene and circulene with a molecular formula C48H24, which can be regarded as an isomer for kekulene, [6,6]carbon nanobelt ([6,6]CNB), [12]cyclacene, and tetrabenzo[8]circulene as well. Infinitene 1 is a bench-stable yellow solid with green fluorescence, and soluble to common organic solvents. The figure-eight molecular structure of 1 was unambiguously confirmed by X-ray crystallography. The scaffold of 1, reminiscent of a squeezed spring, stem from its enclosed, fully-fused architecture, is significantly compressed as manifested by a remarkably shortened distance (3.152–3.192 Å) between the centroids of two π-π stacked central benzene rings and the closest C···C distance of 2.920 Å. Combined lamellar and herringbone-like crystal packing suggested three-dimensional electronic interactions. Fundamental photophysical properties of infinitene 1 were thoroughly elucidated by means of UV-vis absorption and fluorescence spectroscopic studies as well as density functional theory (DFT) calculations. Its configurational stability enabled separation of the corresponding enantiomers (P,P) and (M,M) by a chiral HPLC. Circular dichroism (CD) and circularly polarized luminescence (CPL) measurements revealed that 1 has moderate |gCD| and |gCPL| values.