

Kirika Ueda , Kazuma Amaike , Richard M. Maceiczyk , Kenichiro Itami , and Junichiro Yamaguchi
J. Am. Chem. Soc. 2014, Just Manuscript. DOI: 10.1021/ja508449y
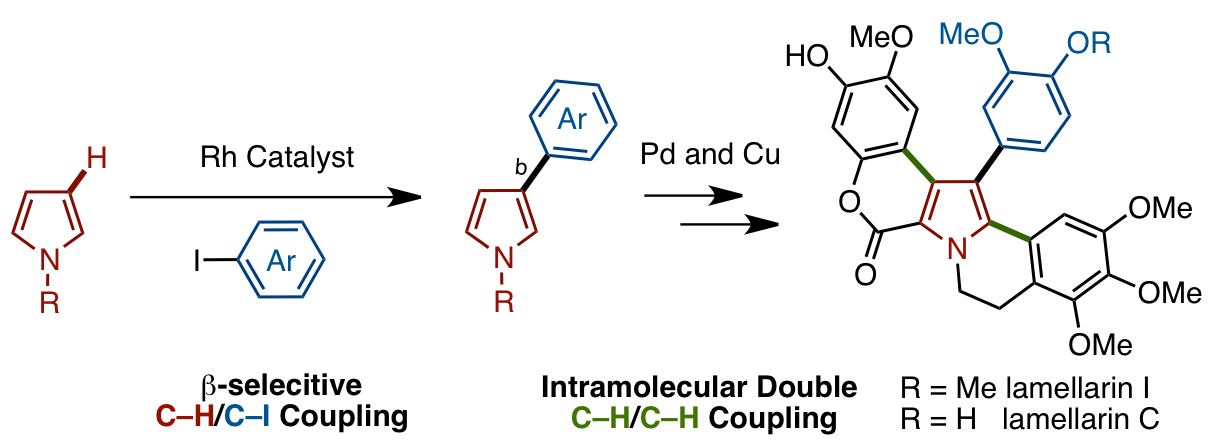
The first general β-selective C–H arylation of pyrroles has been developed by using a rhodium catalyst. This C–H arylation reaction, which is retrosynthetically straightforward but results in unusual regioselectivity, could result in de novo syntheses of pyrrole-derived natural products and pharmaceuticals. As such, we have successfully synthesized polycyclic marine pyrrole alkaloids, lamellarins C and I, by using this β-selective arylation of pyrroles with aryl iodides (C–H/C–I coupling) and a new double C–H/C–H coupling as key steps.