

Huiying Xu, Kei Muto, Junichiro Yamaguchi, Cunyuan Zhao, Djamaladdin G Musaev, and Kenichiro Itami
J. Am. Chem. Soc. 2014, Accepted Manuscript. DOI: 10.1021/ja5071174
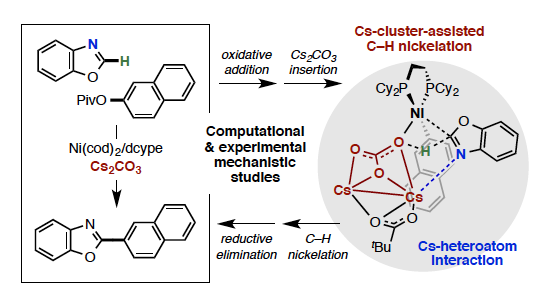
The mechanism of the Ni-dcype catalyzed C–H/C–O coupling of benzoxazole and naphthalen-2-yl pivalate was studied. Special attention was devoted to the base effect in the C–O oxidative addition and C–H activation steps, as well as the C–H substrate effect in the C–H activation step. No base effect in the C(aryl)–O oxidative addition to Ni-dcype was found, but, the nature of the base and C–H substrate plays a crucial role in the following C–H activation. In the absence of base, the azole C–H activation initiated by the C–O oxidative addition product Ni(dcype)(Naph)(PivO), 1B, proceeds via ΔG=34.7 kcal/mol barrier. Addition of Cs2CO3 base to the reaction mixture forms the Ni(dcype)(Naph)[PivOCs.CsCO3], 3_Cs_clus, cluster complex rather than undergoing PivO– → CsCO3– ligand exchange. Coordination of azole to the resulting 3_Cs_clus complex forms intermediate with a weak Cs-heteroatom(azole) bond, the existence of which increases acidity of the activated C–H bond and reduces C–H activation barrier. This conclusion from computation is consistent with experiments showing that the addition of Cs2CO3 to the reaction mixture of 1B and benzoxazole increases yield of C–H/C–O coupling from 32% to 67%, and makes the reaction faster by 3-fold. This emerging mechanistic knowledge was validated by further exploring base and C–H substrate effects via replacing Cs2CO3 with K2CO3 and benzoxazole (1a) with 1H-benzo[d]imidazole (1b) or quinazoline (1c). We proposed the modified catalytic cycle for the Ni(cod)(dcype)-catalyzed C–H/C–O coupling of benzoxazole and naphthalen-2-yl pivalate.