

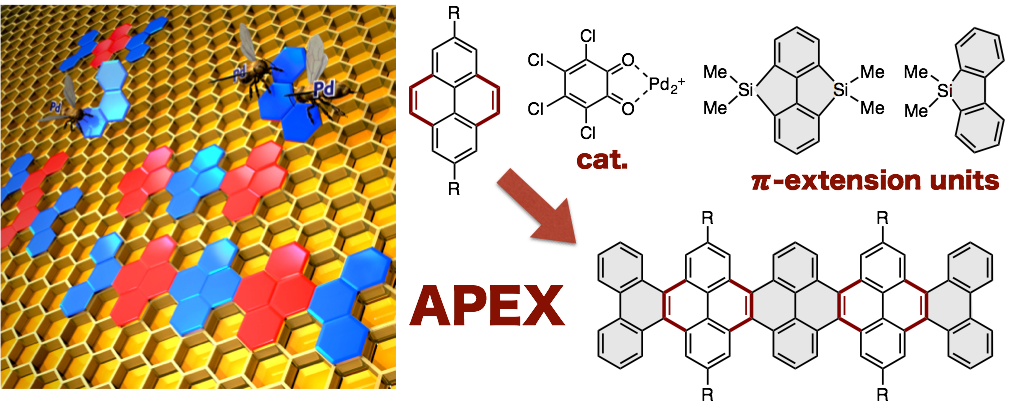
APEX Reaction for Nanographene Synthesis
Kyohei Ozaki, Katsuaki Kawasumi, Mari Shibata, Hideto Ito, Kenichiro Itami
Nature Commun. 2015, 6, 6251. DOI: 10.1038/ncomms7251.
The optoelectronic nature of two-dimensional sheets of sp2-hydridized carbons (for example, graphenes and nanographenes) can be dramatically altered and tuned by altering the degree of p-extension, shape, width and edge topology. Among various approaches to synthesize nanographenes with atom-by-atom precision, one-shot annulative p-extension (APEX) reactions of polycyclic aromatic hydrocarbons hold significant potential not only to achieve a ‘growth from template’ synthesis of nanographenes, but also to fine-tune the properties of nanographenes. Here we describe one-shot APEX reactions that occur at the K-region (convex armchair edge) of polycyclic aromatic hydrocarbons by the Pd(CH3CN)4(SbF6)2/ o-chloranil catalytic system with silicon-bridged aromatics as p-extending agents. Density functional theory calculations suggest that the complete K-region selectivity stems from the olefinic (decreased aromatic) character of the K-region. The protocol is applicable to multiple APEX and sequential APEX reactions, to construct various nanographene structures in a rapid and programmable manner.
Press release, English (Nagoya Univ., WPI-ITbM, JST-ERATO)