

K. Ueda, S. Yanagisawa, J. Yamaguchi, K. Itami
Angew. Chem. Int. Ed. 2010, ASAP. DOI; 10.1002/anie.201005082
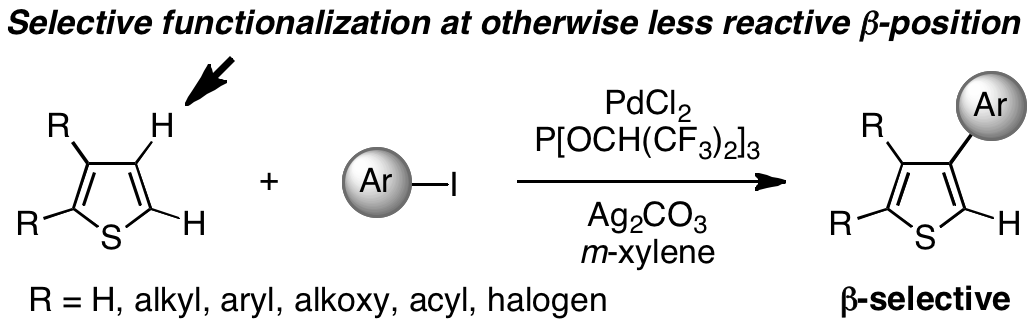
β-Selective C–H Bond Arylation of Thiophenes
The normally less reactive β position of thiophenes was previously inaccessible to direct functionalization. However, the β selectivity observed with the catalytic system PdCl2/P{OCH(CF3)2}3/Ag2CO3 in the arylation of thiophenes with iodoarenes (see scheme) is a remarkably general phenomenon applicable to unsubstituted, monosubstituted, and disubstituted thiophene derivatives, as well as thiophene-containing fused aromatic compounds.