

Eva Koch, Ryosuke Takise, Armido Studer, Junichiro Yamaguchi and Kenichiro Itami
Chem. Commun., 2014, Accepted Manuscript DOI: 10.1039/C4CC08426H
Esters and Amides are available!
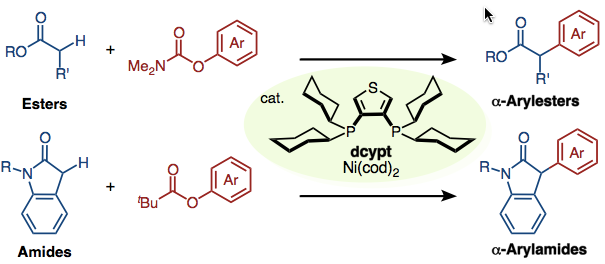
nickel-catalyzed α-arylation of esters and amides with phenol derivatives has been accomplished. In the presence of our unique nickel catalyst, prepared in situ from Ni(cod)2 and 3,4-bis(dicyclohexylphosphino)thiophene (dcypt), and K3PO4, various esters and amides undergo α-arylation with O-arylpivalates or O-arylcarbamates to afford the corresponding coupling products. The thus-obtained α-aryl esters and amides are useful precursors of privileged motifs such as α-arylcarboxylic acids and β-arylamines.