

Yuanming Li, Akiko Yagi, Kenichiro Itami
Chem. Sci., 2019, Accepted Manuscript. DOI:10.1039/c9sc00334g

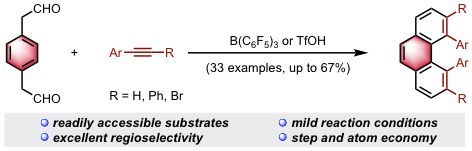
The synthesis of sterically hindered phenanthrenes via acid-catalyzed bisannulation reaction is described. Treatment of 1,4-benzenediacetaldehyde with terminal aryl alkynes in the presence of B(C6F5)3 provides 4,5-diarylphenanthrenes in good yields with excellent regioselectivity. The use of internal alkyne substrates enabled the synthesis of sterically hindered 3,4,5,6-tetrasubstituted phenanthrenes displaying augmented backbone helicity. Furthermore, 1,5-disubstituted, 1,8-disubstituted, 1,2,5,6-tetrasubstituted, and 1,2,7,8-tetrasubstituted phenanthrenes can be obtained through the reaction of alkynes with 1,3-benzenediacetaldehyde or 1,2-benzenediacetaldehyde disilyl acetal.