

Kazuma Amaike, Kei Muto, Junichiro Yamaguchi, and Kenichiro Itami
J. Am. Chem. Soc. 2012, ASAP. DOI: 10.1021/ja306062c
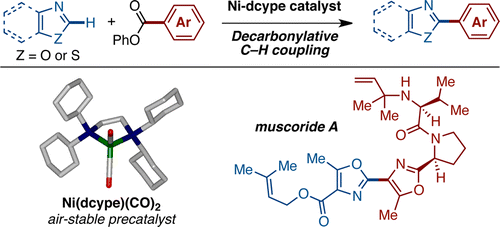
A nickel-catalyzed decarbonylative C−H biaryl coupling of azoles and aryl esters is described. The newly developed catalytic system does not require the use of expensive metal catalysts or silver- or copper-based stoichiometric oxidants. We have successfully applied this new C−H arylation reaction to a convergent formal synthesis of muscoride A.