

Diversity-oriented synthesis of nanographenes enabled by dearomative annulative π-extension
Wataru Matsuoka, Hideto Ito,* David Sarlah,* Kenichiro Itami*
Nature Commun. 2021, 12, 3940. DOI: 10.1038/s41467-021-24261-y [open access]
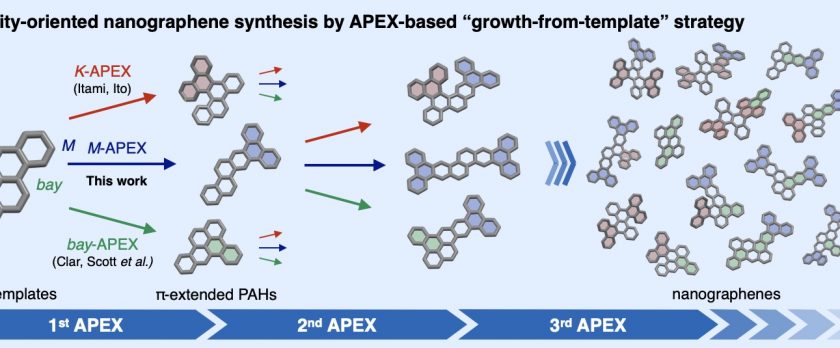
Nanographenes and polycyclic aromatic hydrocarbons (PAHs) are among the most important classes of compounds, with potential applications in nearly all areas of science and tech- nology. While the theoretically possible number of nanographene structures is extraordinary, most of these molecules remain synthetically out of reach due to a lack of programmable and diversity-oriented synthetic methods, and their potentially huge structure-property diversity has not been fully exploited. Herein we report a diversity-oriented, growth-from-template synthesis of nanographenes enabled by iterative annulative π-extension (APEX) reactions from small PAH starting materials. The developed dearomative annulative π-extension (DAPEX) reaction enables π-elongation at the less-reactive M-regions of PAHs, and is suc- cessfully combined with complementary APEX reactions that occur at K- and bay-regions to access a variety of previously untapped nanographenes.