

Jean Bouffard and Kenichiro Itami Org. Lett. 2009, 11, ASAP.
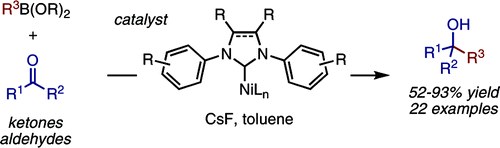
A Ni(cod)2/IPr catalyst promotes the intermolecular 1,2-addition of arylboronate esters to unactivated aldehydes and ketones. Diaryl, alkyl aryl, and dialkyl ketones show good reactivity under mild reaction conditions (≤80 °C, nonpolar solvents, no strong base or acid additives). A dramatic ligand effect favors either carbonyl addition (IPr) or C−OR cross-coupling (PCy3) with aryl ether substrates. A Ni(0)/Ni(II) catalytic cycle initiated by the oxidative cyclization of the carbonyl substrate is proposed.