

Masaki Ohta, Matthias P. Quick, Junichiro Yamaguchi, Bernhard Wünsch, and Kenichiro Itami Chem. Asian J. ASAP.
New way to benzylic amines!
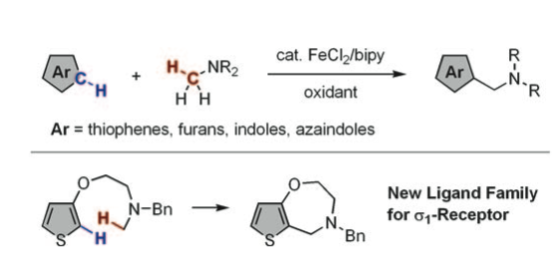
Fe-catalyzed oxidative cross-coupling of electron-rich heteroarenes and methylamines is established. Under the influence of FeCl2/bipy catalyst and oxidant (pyridine N-oxide or H2O2), thiophenes, furans, indoles, and azaindoles cross-couple with methylamines to furnish the corresponding benzylic amines. An intramolecular C H/C
H/C H coupling of a thiophene is observed to afford a good ligand for the
H coupling of a thiophene is observed to afford a good ligand for the  1 receptor protein.
1 receptor protein.