

Shuichi Yanagisawa, Kirika Ueda, Hiromi Sekizawa and Kenichiro Itami, J. Am. Chem. Soc., 2009, ASAP.
DOI: 10.1021/ja906215b
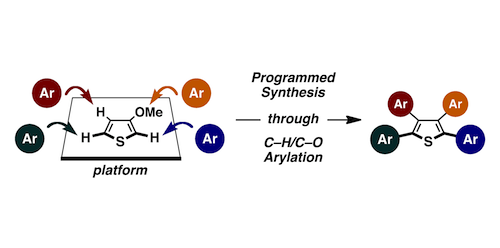
A general protocol for the programmed synthesis of tetraarylthiophenes has been established. The utilization of three catalysts, RhCl(CO){P[OCH(CF3)2]3}2, PdCl2/P[OCH(CF3)2]3, and PdCl2/bipy, enables regioselective sequential arylations at the three C−H bonds of 3-methoxythiophene with iodoarenes. Interesting metal- and ligand-controlled regiodivergent C−H arylations have been uncovered during this study. The installation of fourth aryl groups to the thus-generated 2,4,5-triaryl-3-methoxythiophenes has been accomplished through a sequence of demethylation, triflation, and Suzuki−Miyaura coupling.