

Debashis, Mandal, Atsushi, D. Yamaguchi, Junichiro, Yamaguchi, Kenichiro Itami,
J. Am. Chem. Soc.2011, in press. DOI: 10.1021/ja209945x
Rapidly assemble simple building blocks!
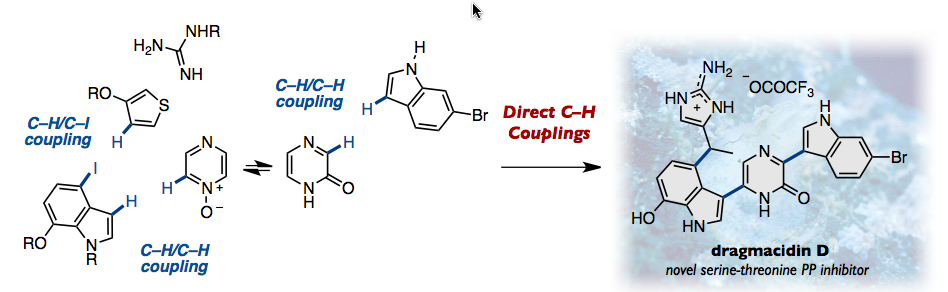
Dragmacidin D, an emerging biologically active marine natural product, has attracted attention as a lead com- pound for treating Parkinson’s and Alzheimer’s diseases. Prominent structural features of this compound are the two indole–pyrazinone bonds and the presence of a polar aminoim- idazole unit. We have established a concise total synthesis of dragmacidin D using direct C–H coupling reactions. Methodo- logical developments include (i) Pd-catalyzed thiophene– indole C–H/C–I coupling, (ii) Pd-catalyzed indole–pyrazine N-oxide C–H/C–H coupling, and (iii) acid-catalyzed indole– pyrazinone C–H/C–H coupling. These regioselective catalytic C–H couplings enabled us to rapidly assemble simple building blocks to construct the core structure of dragmacidin D in a step-economical fashion.