

Hiroki Kamiya, Shuichi Yanagisawa, Satoru Hiroto, Kenichiro Itami, and Hiroshi Shinokubo
Org. Lett. 2011, ASAP. DOI: 10.1021/ol2026069
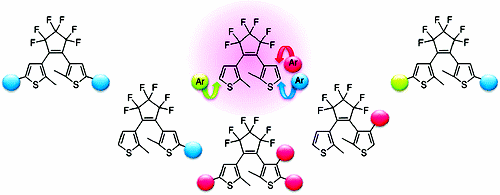
The direct arylation on the thienyl groups of a diarylethene with various aryl iodides efficiently provided arylated dithienylethenes under palladium catalysis. Unsymmetrically substituted dithienylethenes were also synthesized by this protocol. This procedure allows a rapid access to a variety of aryl-substituted dithienylethenes from a single substrate of a simple dithienylethene.